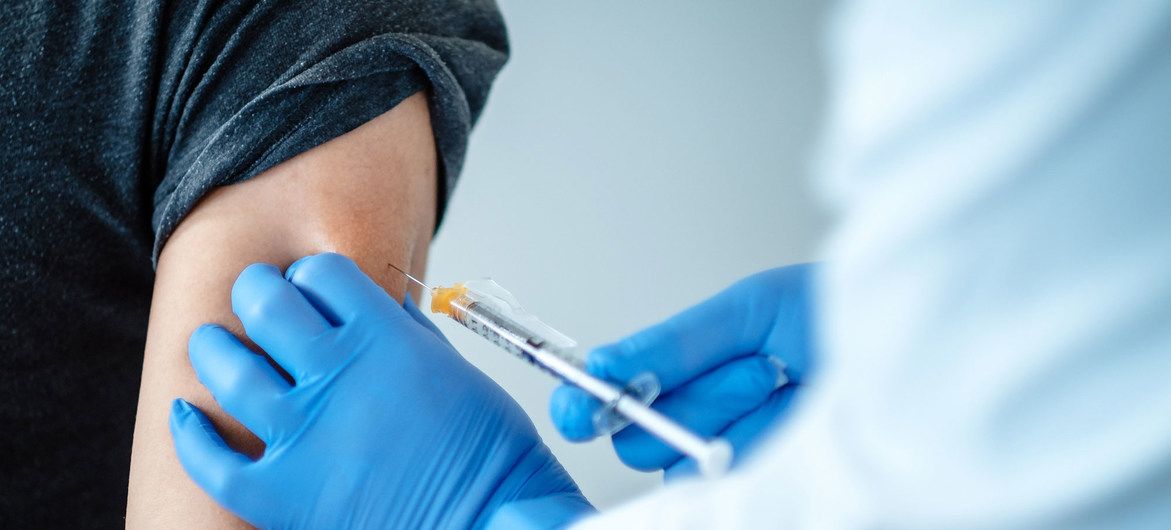
Communications thanks Sopegno, and confirms the new coach
The Cremas Soccer Team in Guatemala Makes Coaching Change After a series of poor results, the Cremas
Solidarity Hug: Protest against Budget Cuts Threatening National Universities and Hospitals
Doctors, students, teachers, and patients come together for a “hug of solidarity” in front of Hospital de
With two goals from Borja, River Plate beat Rosario Central 2-1 and was one step away from the quarterfinals of the League Cup
“,”type”:”raw_html”},{“_id”:”THXJZDO4ONHWXJFCDXQIKIENZA”,”additional_properties”:{},”embed”:{“config”:{“data”:{“date”:”07/04/2024″,”dateToShow”:”07/04/24″,”hour”:”22:58″,”isPinned”:false,”timestamp”:1712541481111}},”id”:”1712541481111″,”url”:” Se juegan cinco minutos más”,”type”:”text”},{“_id”:”EFDHQS53HRBM5LIGGXX57UYMVI”,”additional_properties”:{},”embed”:{“config”:{“data”:{“date”:”07/04/2024″,”dateToShow”:”07/04/24″,”hour”:”22:55″,”isPinned”:false,”timestamp”:1712541336215}},”id”:”1712541336215″,”url”:” ¡GOL DE RIVER PLATE!”,”type”:”text”},{“_id”:”2C7IC6XCTBCV7F5QQA52DXVKPU”,”additional_properties”:{},”content”:”Miguel Borja definió tras un gran pase de
Popular Stories

Maripily Rivera Nominated Once Again in The House of the Famous
Puerto Rican Maripily Rivera faces elimination once again in the fourth season of The House of the

Communications thanks Sopegno, and confirms the new coach
The Cremas Soccer Team in Guatemala Makes Coaching Change After a series of poor results, the Cremas
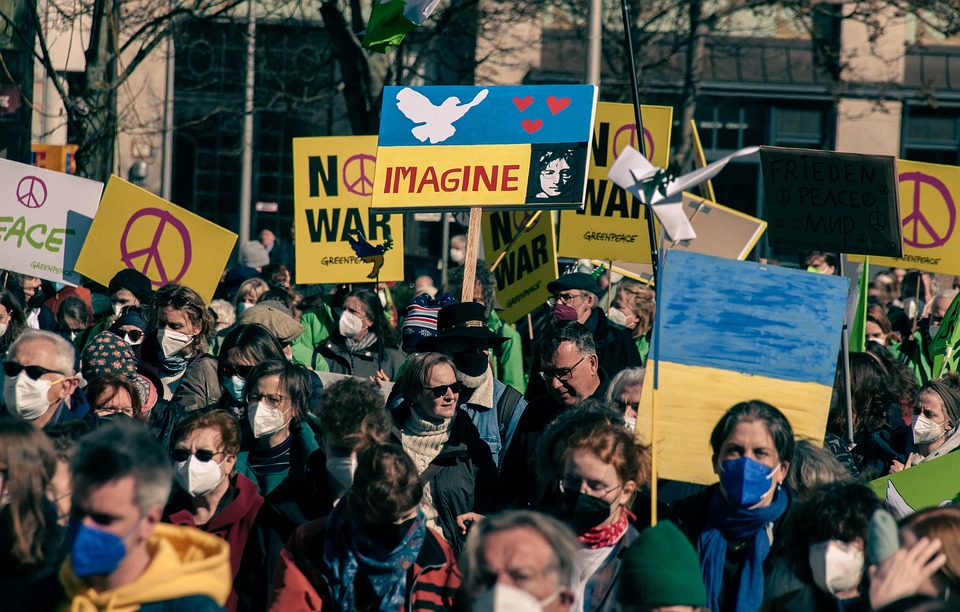
Solidarity Hug: Protest against Budget Cuts Threatening National Universities and Hospitals
Doctors, students, teachers, and patients come together for a “hug of solidarity” in front of Hospital de
With two goals from Borja, River Plate beat Rosario Central 2-1 and was one step away from the quarterfinals of the League Cup
“,”type”:”raw_html”},{“_id”:”THXJZDO4ONHWXJFCDXQIKIENZA”,”additional_properties”:{},”embed”:{“config”:{“data”:{“date”:”07/04/2024″,”dateToShow”:”07/04/24″,”hour”:”22:58″,”isPinned”:false,”timestamp”:1712541481111}},”id”:”1712541481111″,”url”:” Se juegan cinco minutos más”,”type”:”text”},{“_id”:”EFDHQS53HRBM5LIGGXX57UYMVI”,”additional_properties”:{},”embed”:{“config”:{“data”:{“date”:”07/04/2024″,”dateToShow”:”07/04/24″,”hour”:”22:55″,”isPinned”:false,”timestamp”:1712541336215}},”id”:”1712541336215″,”url”:” ¡GOL DE RIVER PLATE!”,”type”:”text”},{“_id”:”2C7IC6XCTBCV7F5QQA52DXVKPU”,”additional_properties”:{},”content”:”Miguel Borja definió tras un gran pase de
Travel & Explore the world

Maripily Rivera Nominated Once Again in The House of the Famous
Puerto Rican Maripily Rivera faces elimination once again in the fourth season of The House of the

Communications thanks Sopegno, and confirms the new coach
The Cremas Soccer Team in Guatemala Makes Coaching Change After a series of poor results, the Cremas
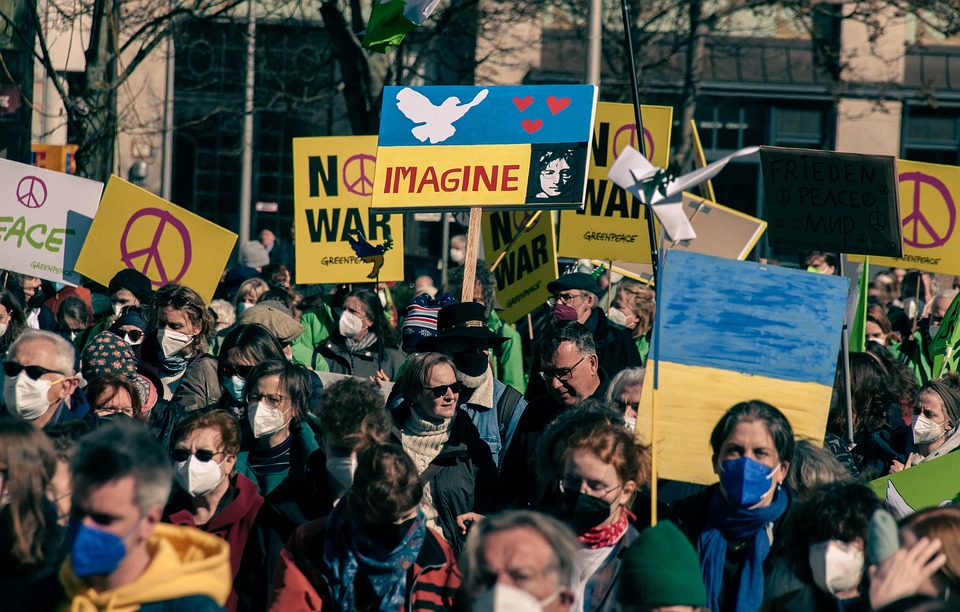
Solidarity Hug: Protest against Budget Cuts Threatening National Universities and Hospitals
Doctors, students, teachers, and patients come together for a “hug of solidarity” in front of Hospital de
With two goals from Borja, River Plate beat Rosario Central 2-1 and was one step away from the quarterfinals of the League Cup
“,”type”:”raw_html”},{“_id”:”THXJZDO4ONHWXJFCDXQIKIENZA”,”additional_properties”:{},”embed”:{“config”:{“data”:{“date”:”07/04/2024″,”dateToShow”:”07/04/24″,”hour”:”22:58″,”isPinned”:false,”timestamp”:1712541481111}},”id”:”1712541481111″,”url”:” Se juegan cinco minutos más”,”type”:”text”},{“_id”:”EFDHQS53HRBM5LIGGXX57UYMVI”,”additional_properties”:{},”embed”:{“config”:{“data”:{“date”:”07/04/2024″,”dateToShow”:”07/04/24″,”hour”:”22:55″,”isPinned”:false,”timestamp”:1712541336215}},”id”:”1712541336215″,”url”:” ¡GOL DE RIVER PLATE!”,”type”:”text”},{“_id”:”2C7IC6XCTBCV7F5QQA52DXVKPU”,”additional_properties”:{},”content”:”Miguel Borja definió tras un gran pase de

How Manchester City’s homegrown Phil Foden became the “best player in the Premier League” – Champions League
Manchester – There aren’t many people left at Manchester City who have been with the club longer
[Notice]For customers from the European Economic Area (EEA) and the United Kingdom – Yahoo! JAPAN
From Wednesday, April 6, 2022, Yahoo! JAPAN is no longer available in the EEA and the United

Kristoffer Klaesson, Car accident | English newspaper: – Leeds confirms Norwegian football pro in car accident
On Wednesday morning there were reports that a prominent Norwegian footballer had been involved in an accident