This article is reproduced from CASE Science News “The entire universe is my zoo-Astronomy and Chemistry”
The entire universe is like a “molecular zoo”. By studying the molecular spectrum, we can know the molecular distribution, temperature and other properties; and because different molecules have different “habits,” we can also know that these The molecular interstellar environment.
AstrochemistryWhat is it?
Astronomy is the natural science that studies the celestial bodies in the universe. In addition to the “astrophysics” that the general public knows better, the universe has many aspects, one of which is the theme of this article: “Astrochemistry.”
Both are sciences that study “materials,” but physics and chemistry observe the world in different ways. Astrochemistry focuses on the “atoms, molecules, and ions in different celestial environments” in the universe, and studies their formation, distribution, interaction with each other, or interaction with the environment. (In the following, for convenience, we will collectively refer to molecules, ions, etc. as molecules.)
Although astronomy is one of the oldest sciences, the subject of astrochemistry did not begin to appear until the middle of the 20th century. The reason is simple: because the molecules cannot be seen! A star is such a big one that you may not be able to see it clearly with a telescope, let alone a molecule that you can’t see before your eyes?
Therefore, to study molecules in the universe, we must rely on special techniques; among them, one of the most important techniques is “spectroscopy.”
Spectrum is a pattern that arranges light according to wavelength or frequency. Like a “rainbow” is a kind of spectrum, which is a pattern of sunlight separated according to different frequencies. In addition to visible light, there are many wavelengths invisible to the naked eye, such as radio waves, infrared rays, ultraviolet rays, X-rays, and so on.
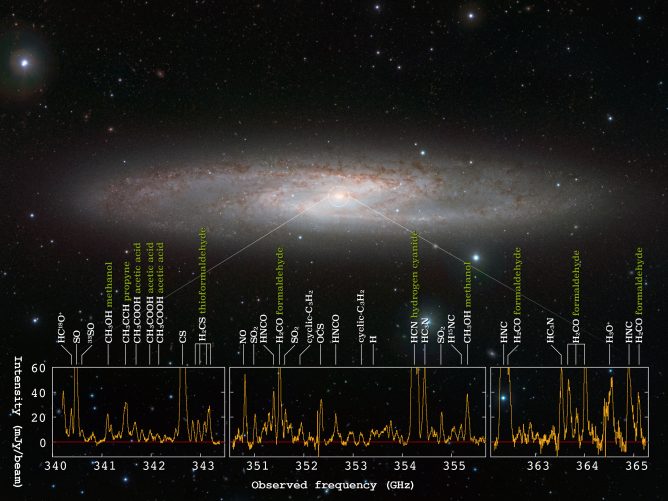
Every molecule has its own spectrum. If we on earth want to know what the spectrum of a molecule looks like, in addition to experimental measurements, we can use computers to do precise simulation calculations to predict. The spectra of molecules are like their “fingerprints”, just as the police compare the collected fingerprints with a database to find out who left the fingerprint. Astronomers compare the observed spectra with the database. Compare, to know which molecules are on the other side of the distant interstellar, even their content, temperature, etc. (Figure 1).
To learn more about how astronomers use spectroscopy, you can refer to: “Take the Light apart: Spectra in Astronomy”.
Why the universe is“Molecular Zoo”?
animalsIt can often reflect the local environment. For example, when you see a hippopotamus, you know that there is an environment with water and grass; when you see sakura salmon, you know that there is a stream with low water temperature. [3].Treat the universe as a molecular animalThe same is true for gardens. Observing the distribution and content of molecules can also allow us to infer the physical environment. At present, we have observed more than 200 kinds of molecules from the interstellar space. Here are some common interstellar molecules!

Molecular hydrogen, H2)
The most abundant molecules in the universe are also the main components of the “molecular cloud.” There are approximately 10,000 hydrogen molecules per cubic centimeter in the molecular cloud (104 cm-3)。
Molecular clouds are places where stars and planets are born, so understanding the distribution of hydrogen molecules can help us study star formation. At the same time, hydrogen molecules can react with heavier elements and are catalysts for many chemical reactions, producing other molecules such as carbon monoxide (CO) and carbon dioxide (CO).2), cyano radical (CN), etc.
Hydrogen molecule is very important to astrochemistry. Unfortunately, it is almost impossible to observe it in an environment where the average temperature of molecular cloud is only more than minus 200 degrees (because it is a symmetric molecule, interested readers can learn more. )[5][6]
Carbon monoxide (CO)
Carbon monoxide is distributed in the interstellar areas with low temperature and high density. It is the second most interstellar molecule.
Compared with hydrogen molecules, carbon monoxide is easier to observe too much, so it is easier for astronomers to know the distribution of molecular clouds from carbon monoxide images. Since molecular clouds are almost impossible to observe directly with visible light, early scientists had no idea that there were so many molecular clouds around us. They did not open their eyes until after observing the carbon monoxide image. [5][6][7]

Ammonia (ammonia, NH3)
Ammonia is also easily observed as a molecule. The first molecule observed in history was ammonia. Ammonia has many spectral lines, and the intensity of these spectral lines is very sensitive to environmental changes and can correspond to many different interstellar environments.Observation of ammonia allows us to more accurately derive the environmental conditions there [8][9]。
The environment in the universe has changed too much, and the chemical reactions in different environments may be very different. In the universe, the diffuse cloud, dense cloud, hot core formed by stars, and other regions where a large number of molecules have been detected, the temperature distribution ranges from 10 K to 1000 K ( About -200 degrees to +800 degrees Celsius), the density ranges from one hundred particles per cubic centimeter to ten trillion particles (102 cm-3~1013 cm-3) Both!
Here we will introduce several interstellar environments with high molecular content.
Star-forming region
High density inside the molecular cloud where stars are forming. The Orion KL Nebula (Orion KL) is the most active area of star formation in the Orion macromolecular cloud. There are many “complex saturated organic molecules” appear here, such as: methanol (CH3OH), methyl formate (HCOOCH3), etc., there are also some long-chain carbon molecules, such as: cyano acetylene (HCCCN)[10]。

Comet 67P / Churyumov-Gerasimenko (comet 67P / CG)
In the observational data in recent years, scientists have seen extremely high levels of molecular oxygen (O2), which surprised them very much.Because oxygen molecules can easily react and become other molecules in the universe, but in a volatile environment like a comet, there can be a high content of oxygen molecules, which means that these oxygen molecules are likely to be formed in the comet. At that time, it already exists in the surrounding environment and is frozen on the comet [11][12]。
The scope of astrochemistry involved is very wide, spanning many different fields. The entire universe is a “molecular zoo.” Astronomers observe the molecules in these universes to learn what kind of environment exists in distant celestial bodies. Many organic molecules have also been discovered in the interstellar space. Studying these molecules can even help us understand the origin of life. This is now a key direction of astrochemical research.
Reference

