Loading player
Earlier this week, a drug was approved in the United States to treat alopecia areata, a disease that can cause total loss of hair and other body hair, including nose and ear, eyelashes and eyebrows. The drug, the molecule of which is called baricitinib, was developed against rheumatoid arthritis by the pharmaceutical company Eli Lilly, but has shown to have potential in the treatment of alopecia areata and possibly other dermatological diseases.
Approval by the Food and Drug Administration (FDA), the US government agency that deals with drug safety, will make it possible to use baricitinib for purposes other than the treatment of rheumatoid arthritis, giving patients reimbursements from part of the insurance, since the treatment costs around $ 2,500 a month. Two other similar drugs, developed by Concert Pharmaceuticals and Pfizer, may soon be approved by the FDA, offering additional possibilities for people with alopecia areata.
In general, alopecia is the process that leads to a reduction in the quantity and quality of hair, possibly until it disappears. The causes of the disease are not yet fully known, but studies carried out so far indicate hereditary, hormonal and immune components. The most common form, and which mainly involves men, is androgenetic alopecia where, due to hereditary factors, the hair bulbs tend to become smaller and smaller over time, leading to the formation of increasingly thin and fragile hair, which falls out causing a thinning more and more evident.
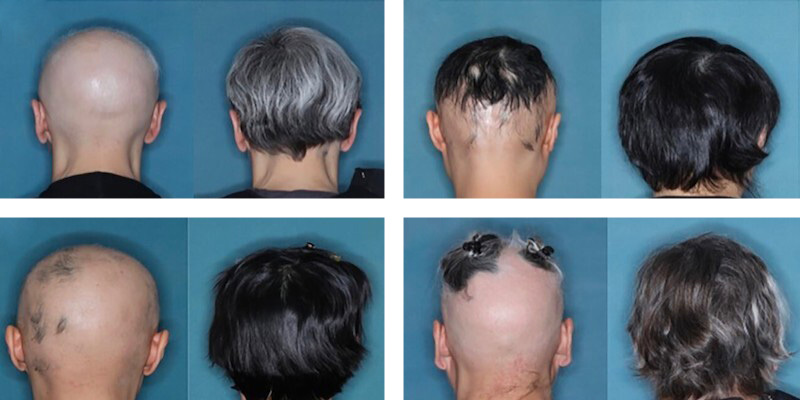
Alopecia areata, on the other hand, manifests itself with a more sudden loss of hair and other body hair, causing rather evident hairless patches. In many cases it resolves spontaneously and without leaving marks, but in some people the disease extends to the entire scalp and other hair bulbs, leading to total loss of hair. The condition has been studied for some time and it is believed that the root cause may be an unexpected reaction of the immune system, which locally damages the hair bulbs.
Baricitinib is an inhibitor of JAK, a family of enzymes that is involved in certain activation processes of the immune system. Inhibitors of this type are considered good candidates for treating some forms of tumors and especially some autoimmune diseases caused by an off-scale response from the immune system, as in the case of rheumatoid arthritis.
The use of JAK inhibitors in the dermatological field is mainly attributed to Brett King, a dermatologist at Yale University (United States) who in the early 1910s had come across some studies carried out on mice, where a role was evident of these inhibitors in successfully treating cases of alopecia areata. King had then started some clinical trials involving his own patients, and had obtained very encouraging results on the possibility of restoring the scalp damaged by the disease.
His early studies on the subject had attracted the attention of other research groups, sparking the interest of some pharmaceutical companies such as Eli Lilly, the company that developed the now FDA-approved baricitinib.
The approval was made possible following the analysis of two clinical trials, funded by Eli Lilly, and the results of which were published in May in the New England Journal of Medicine. The tests involved 1,200 patients with severe forms of alopecia areata. A little over 8 months after the start of treatment, 40 percent of the participants reported a partial or complete regrowth of hair and other hairs as appropriate. After a year, about half of the patients returned to having hair in amounts comparable to before the disease.
During the trial, no worrying side effects were reported. Some volunteers indicated that they had some acne or some infections, especially of the urinary tract. In many cases, the adverse effects resolved on their own over time, without the need to combine other treatments with JAK inhibitors.
The clinical trials were conducted under the supervision of King, who is also involved in the trials Pfizer and Concert Pharmaceuticals are running. If the new tests lead to similar results, and further FDA approvals, patients who do not get results with one drug will be able to try another, increasing the chances of success in treating alopecia areata.
JAK inhibitors are part of a very active area of pharmacological research and in recent years have led to the approval of various drugs and their use for the experimental treatment of diseases for which they were not initially developed. As with all recently approved drugs, however, it is good to keep some caution in evaluating the benefits, which as always can vary significantly from person to person.