Junshi Bio (688180.SH) has released two consecutive announcements on the progress of clinical trials of its oral anti-new coronavirus drug VV116.
On the evening of March 15, Junshi Bio announced that the oral nucleoside anti-SARS-CoV-2 drug VV116 tablets (hereinafter referred to as VV116), jointly developed by the company’s holding subsidiary Juntuo Bio and Wangshan Wangshui, has launched a A Phase III clinical study was completed, and the enrollment and administration of the first patient were completed. The Phase III clinical study aims to evaluate the efficacy and safety of VV116 in the treatment of patients with moderate to severe novel coronavirus pneumonia compared with standard treatment. It is an international multi-center randomized, double-blind, controlled study.
On March 16, Junshi Biosciences disclosed another announcement on the release of 3 Phase I clinical data of VV116. According to the announcement, Acta Pharmaceutica Sinica, a well-known journal in the field of pharmacy, published the results of three Phase I clinical studies of the oral nucleoside anti-SARS-CoV-2 drug VV116. The results of the study showed that VV116 showed satisfactory safety and tolerability in healthy subjects, and it was rapidly absorbed orally and could be administered orally under fasting or normal diet conditions. It is recommended to explore two daily doses in follow-up clinical studies. Administer doses of 200 mg to 600 mg each time. This is the first time that a domestic oral small molecule anti-SARS-CoV-2 drug has published Phase I clinical data.
According to a material provided by Junshi Bio to the reporter of “Daily Economic News”, the three Phase I clinical studies published by VV116 were all led by Director Liu Gangyi and Director Yu Chen of Shanghai Xuhui District Central Hospital as the principal investigators. . Among them, Study 1 (NCT05227768) and Study 2 (NCT05201690) were randomized, double-blind, placebo-controlled, single-dose and multiple ascending dose studies to evaluate the safety of single and multiple ascending oral VV116 in healthy subjects , tolerability, and pharmacokinetic characteristics; Study 3 (NCT05221138) was a randomized, open-label, 3-cycle, crossover study to observe the effect of diet on the pharmacokinetics and safety of oral VV116 in healthy subjects .
Between November 2021 and January 2022, the study enrolled a total of 86 eligible adult healthy subjects, with 38 subjects in Study 1, 36 subjects in Study 2, and 12 subjects in Study 3. tester.
research shows:
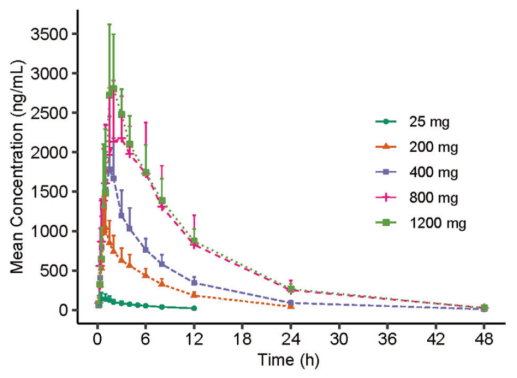
(1) VV116 is rapidly absorbed orally.In a single ascending dose study, oral VV116 can be rapidly hydrolyzed to the active metabolite 116-N1, and the average plasma drug peak time (Tmax) is only 1.00 hours to 2.50 hours. In addition, the mean half-life (t 1/2) value of 116-N1 was 4.80 hours to 6.95 hours, suggesting that a twice-daily (BID) dosing regimen can be explored in clinical treatment.
Figure 1: Mean concentration-time curves of 116-N1 in plasma of each dose group after single administration
(2) Repeated administration can maintain effective antiviral concentration.In a multiple ascending dose study, administered twice daily (12 hours apart) for 5.5 days (days 1 to 6), in vivo drug concentrations were available in all three dose groups (200 mg, 400 mg, and 600 mg). Maintained above the effective antiviral level (trough concentration greater than EC90 against Omicron variant).

Figure 2: Mean Concentration-Time Profiles of 116-N1 in Plasma on Days 1 and 6 in Multiple Escalating Dose Study
(3) Ordinary diet had no effect on VV116 drug exposure.Median Tmax under fasting, normal diet, and high-fat diet conditions were 1.50 hours, 3.00 hours, and 2.50 hours, respectively, suggesting that diet prolongs the time to peak drug but does not affect peak drug concentrations. A high-fat diet slightly increased the area under the plasma concentration-time curve (AUC), and it is recommended that oral drug therapy be administered on an empty stomach or under normal dietary conditions.
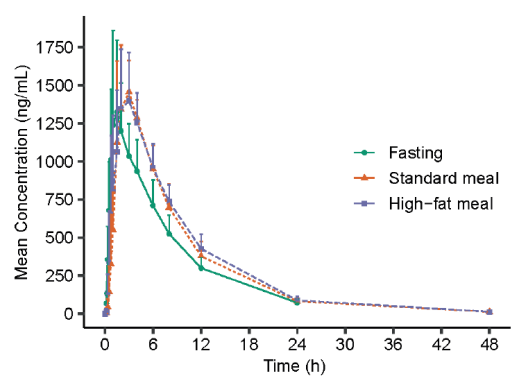
Figure 3: Mean concentration-time profiles of 116-N1 in plasma under fasting and fed conditions
In terms of safety, VV116 showed satisfactory safety and tolerability in healthy subjects. None of the three studies reported death, serious adverse events (SAEs), grade 3 or higher adverse events (AEs), or AEs leading to discontinuation or discontinuation of treatment. All AEs recovered without treatment or intervention. Compared with previously reported data for similar drugs, VV116 has a lower risk of hepatotoxicity.
Based on the positive results of the VV116 Phase I study, Junshi Biosciences and Wangshan Wangshui have initiated an international multicenter, double-blind, randomized, placebo-controlled, Phase II/III clinical study (NCT05242042) in the treatment of mild to moderate COVID-19 patients. 19 patients. The study, co-chaired by Professor Zhang Wenhong from Huashan Hospital Affiliated to Fudan University and Professor Shen Yinzhong from Shanghai Public Health Clinical Center, aimed to evaluate the efficacy, safety and pharmacokinetics of VV116 for early treatment of mild to moderate COVID-19 patients. In addition, another international multicenter, randomized, double-blind, controlled phase III clinical study evaluating the efficacy and safety of VV116 in moderate to severe COVID-19 patients is ongoing.
Source of cover image: Photo Network-500327248