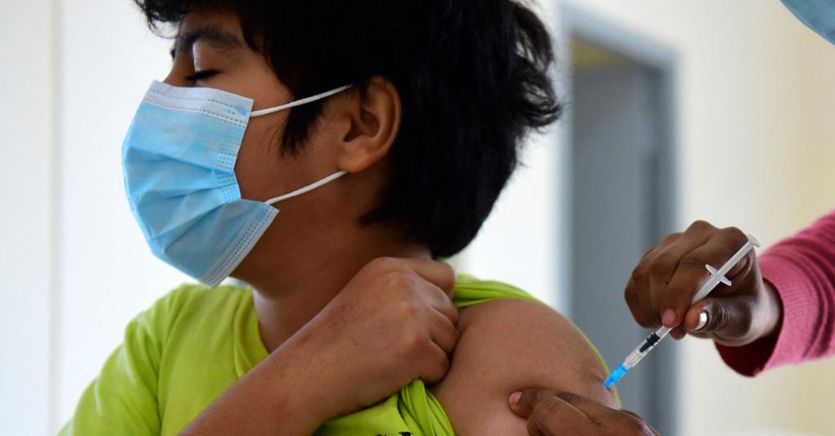
Pfizer and BioNTech announced on September 20 the positive results of testing their Covid-19 vaccine in children between 5 and 11 years of age. The results of phase 2 and 3 studies, the two companies report, have shown a favorable safety profile and solid neutralizing antibody responses in children between the ages of 5 and 11: in other words, the drug proved to be “safe, well tolerated “and produced a” robust “immune response in that age group, compared to dosages lower than those required for older recipients.
The tests used a two-dose regimen of 10 micrograms given 21 days apart – less than the 30 microgram dose used for people 12 years of age and older.
Loading…
Towards the request for the green light to Fda and Ema
Antibody responses in participants who were given doses of 10 micrograms were comparable to those recorded in a previous Pfizer-BioNTech study in people aged 16 to 25 years immunized with doses of 30 micrograms. The 10 microgram dose was selected as the preferred dose for safety, tolerability and immunogenicity in children aged 5 to 11 years.
Pfizer and BioNTech plan to share this data with the US Food and Drug Administration (Fda), the European Medicines Agency (EMA) and other regulatory authorities as soon as possible.
For the U.S., companies expect to include the data in a short-term Emergency Use Authorization (Eua) submission as they continue to accumulate the safety and efficacy data needed to submit full FDA approval in this. age range.