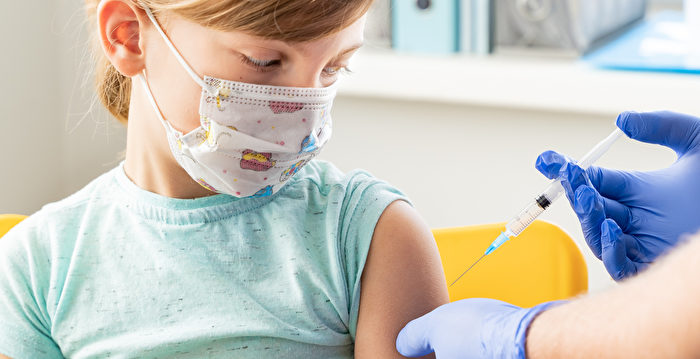
[Epoch Times October 23, 2021](Epoch Times reporter Chen Ting comprehensive report) Experts from the U.S. Food and Drug Administration (FDA) stated on the evening of Friday (October 22) that Pfizer The company’s child-dose COVID-19 vaccine seems to be very effective in preventing infections in elementary school students, and it has not caused unexpected safety issues.
Pfizer said earlier on Friday that in a clinical trial, their vaccine had 90.7% protection for children aged 5 to 11.
The company said that among the 2,268 adolescents who participated in the trial, none of them had a serious illness, but those who were vaccinated had much milder symptoms than those who had not been vaccinated.
FDA scientists analyzed the trial data and stated that in almost all cases, the benefits of the vaccine to prevent hospitalization and death from COVID-19 outweigh the risks of any serious potential side effects. But the agency’s reviewers have not yet requested approval of Pfizer’s vaccine.
Next Tuesday, the FDA will hold a public meeting to discuss whether 28 million children between the ages of 5 and 11 can be vaccinated in the United States. At that time, the FDA will require an external panel of experts to vote on the issue.
If the FDA authorizes injections for elementary school students, Pfizer vaccine will become the first COVID-19 vaccine for this age group. Once passed, it can start in early November, and the Centers for Disease Control and Prevention (CDC) will make additional recommendations on who should be vaccinated in the first week of November.
Both Pfizer and Modena vaccines may cause rare cases of heart inflammation, especially in young men.
Pfizer stated in the report that the incidence of myocarditis in the 5 to 11-year-old age group is lower, possibly because younger children have lower injection doses.
Previously. Pfizer has recommended a full dose of the vaccine for people over 12 years of age. Children between the ages of 5 and 11 will receive two doses of 10 micrograms, which is one-third of those of people 12 years and older.
Pfizer said on Friday that it has expanded clinical trials to determine the safety of the vaccine, and it is said that the number of children participating in the next phase of the trial has more than doubled.
The FDA stated that no new side effects were found after the review. Those side effects that do occur mainly include arm soreness, fever or pain.
However, scientists from the US Food and Drug Administration pointed out that the scale of the study was not large enough to detect extremely rare side effects, including myocarditis, an inflammation of the heart that occasionally occurs after the second dose.
Although the risk of severe illness and death in children is lower than that of the elderly after infection, according to the US CDC, COVID-19 has caused more than 630 Americans aged 18 and under to die.
The American Academy of Pediatrics says that nearly 6.2 million children have been infected with the coronavirus. In the past six weeks, with the surge in Delta variants, more than 1.1 million children have been infected with COVID-19.
The U.S. government has purchased enough child doses for children between 5 and 11 years old across the country. These vaccines are packed in special orange capped vials to distinguish them from adult vaccines. If approved, vaccines and child-specific needles will be quickly sent to various places.
The Associated Press contributed to this report.
Editor in charge: Ye Ziwei#
.