On May 26, the Phase III clinical report of Sinopharm Vaccine was finally published in the Journal of the American Medical Association (JAMA), while the Phase III report of Kexing Vaccine has not yet been officially published through peer review at this time. These two vaccines in China are among the first vaccines in the world to complete Phase III clinical trials and obtain emergency authorization from China. However, the specific results of their Phase III trials were almost released in journals at the latest, which has caused widespread doubts internationally. . Judging from the Phase III test reports of Sinopharm and Kexing Vaccine, there are still two obvious problems.
Why is China’s vaccine phase III clinical results not transparent?
Previously, many international experts pointed out that the Phase III clinical trial data of China Kexing and Sinopharm Vaccine are not transparent and have not been published in journals for a long time. However, many Chinese people are puzzled: the Health Commission has announced that China’s Kexing vaccine has conducted Phase III clinical trials in Brazil and other countries, with a protection rate of about 50%; the protection rate of Sinopharm vaccine is more than 70%, and the results are clear. Chu posing, why did you say “not published”?
Here needs to explain a concept, what is called“The results of Phase III clinical trials are published in journals”。
First of all, what is the “Phase III clinical trial”?
The development of a vaccine requires animal experiments and then human trials.Human trials are divided into several steps: first, do smaller-scale Phase I and II clinical trials, and then doLarger Phase III clinical trial.whenPhase III test results confirm that the vaccine is effective and safeAfter that, the government considered approving the listing and attacking the people.
So what does “published in a journal” mean?
What we usually say about journals isPeer-reviewed journal.In other words, the vaccine’sAfter the results of the third phase trial came out, It needs to be reviewed in academia and certified by other experts in the same field. The purpose is to ensure the effectiveness and safety of the final vaccine announcement, in line with general academic standards, without bias.After the review, the vaccine‘s Phase III trial report will be published in a peer-reviewed journal.
Common international medical authoritative journals, including “The Lancet” (The Lancet), “Journal of the American Medical Association” (JAMA), “New England Journal of Medicine” (NJEM) and so on.
So, as you can see,Pfizer vaccineinLast DecemberAfter completing the Phase III trial, it was reported onCurrent monthPublished in the “New England Journal of Medicine”.Modena vaccineThe report of the third phase of the trial was published in the New England Journal of Medicine on February 4.
Other approved vaccines, such asAZ vaccine, Johnson & Johnson vaccine, Russian satellite V vaccine, The third phase of the trial report has also been published in journals.
however,Sinopharm VaccineAlready inLast DecemberCompleted the interim analysis of the Phase III trial and approved the listing in China.But its Phase III trial medical report, butHasn’t published。
Not only that, but Sinopharm itself has not released a specific Phase III trial report to the public. At that time, the statement of Sinopharm only stated that a vaccine candidate produced by its affiliated Beijing Institute of Biological Products had an effective rate of 79%.The whole statement is only a few sentences, and there is no provision for the third phase of the trialDetailed medical data, Such as: How did the Phase III trial be done? How many people will be vaccinated? The specific ratio of different ages? How many kinds of systemic and local side effects are there? What is the rate of each side effect? How many accidental deaths are there? Is it related to vaccines? ……Etc., etc.
These should usually beNormal vaccine company website, National Center for Disease Control and PreventionmeetingDetailed announcementThe data from the China Vaccine Company website and the China Center for Disease Control and Prevention,Almost blank。
This is why China’s vaccines have been criticized for being opaque.
Only give the people a number.andHow did this number come from——The most critical part has been omitted from the ambiguity.
It wasn’t until four or five months later that Sinopharm slowly released the data. At the end of May, the results of the Phase III clinical trial of Sinopharm Vaccine were published in international journals.
Kexing is the world‘s first vaccine to complete Phase III clinical trials. The results of its three phases are still unpublished.StillPreprint stage(“Preprint” refers to a report that has not yet passed peer review, and is generally not counted as officially published in a journal).
So, where did the data go in these blank months? Even if it is not accepted by international journals, why not publish it to the public and the medical community first? It is difficult for us to know.
Two major problems in the three phase reports of Sinopharm and Kexing
However, the three phase reports of Sinopharm and Kexing Vaccine are finally overdue. Are the results completely relieved? Not at all.
In the finally released report, there are two obvious issues that should be brought to the attention of the public:
1. The population of test subjects, mainly young people
We know that the people who have the highest priority for vaccinations against the new crown vaccine in various countries (except for frontline medical staff) are elderly people over 65 years old. This is because elderly people over 65 are the most vulnerable group to contract the new crown.
Therefore, in Phase III clinical trials, the most important thing is to observe the protection of the vaccine for the elderly.Vaccine companies, All includedA significant proportion of the elderly。
In the Phase III trial of Pfizer vaccine, 42% were elderly people over 55 years old; in the Phase III trial of Modena vaccine, 25% were elderly people over 65 years old. however,Coxing VaccineIn Brazil’s Phase III trial, only recruited5% of the elderly;SinopharmThe published clinical trial site is in the Middle East, recruitedThe elderly are even less than 2%。
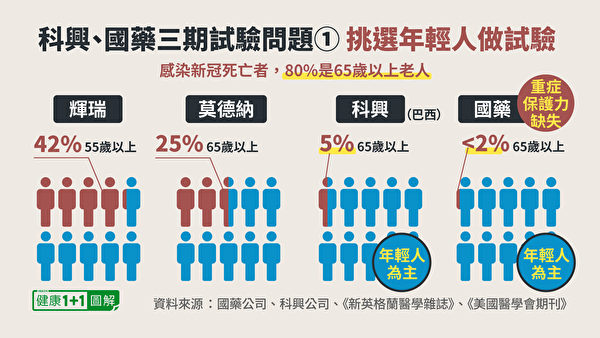
Not only that, but the article on Sinopharm Vaccine also pointed out in the discussion section on the limitations of research:Sinopharm Vaccine Phase III TrialThe main target isHealthy, young Middle Eastern men, And lack of sufficient efficacy to test the effect of the vaccine in the elderly, women, and patients with chronic diseases.
This group of people is the least likely to be infected with the new crown. When the pharmaceutical company knows that the new crown is mainly infected with chronic diseases and the elderly, why do they conduct clinical trials with young people as the mainstay and hardly recruit the elderly? And can the number of vaccine protection obtained from the young population be counted as the true protection of the vaccine? The researchers of Sinopharm themselves also stated that they cannot be directly used to infer the protection of the vaccine against the elderly, men, and patients with chronic diseases.
Obviously, Sinopharm and Kexing have obvious biases when selecting subjects.
2. The side effects of Sinopharm vaccine are lower than adjuvants
There is still a problem with the test report provided by Sinopharm Vaccine, which is that the proportion of side effects is extremely low.
Many people believe that low side effects mean that the vaccine is safe and a good thing. Here we must first clarify a concept.
Side effects occur after administering the vaccine (this is not about serious or abnormal side effects, but aboutGeneral side effects),is normal. This is because when the vaccine enters the body and stimulates an immune response, the body will inevitably experience fever, headache, fatigue and other reactions.This representsThe body’s immune system is being activated, The vaccine is working.
Elderly people who administer the new crown vaccine rarely have side effects because their immune system is already weak. After the vaccine enters the body, it is difficult to stimulate the immunity as large as young people.
From the three phase reports of Pfizer and Modena, it can be seen that the proportion of systemic side effects is more than 70%.Higher than the control placebo. this is normal. At least, the side effects caused by the injection of the vaccine into the human body should generally be higher than the control group.
So, why does the control group, such as normal saline, cause 30% of side effects? Because people don’t know whether they are taking a real vaccine or saline when participating in the experiment, they will have a certain psychological effect.
In the control group of Sinopharm vaccine, adjuvants are used (adjuvants are auxiliary components of the vaccine and do not contain the main component-inactivated virus, which does not stimulate an immune response by itself). The proportion of side effects caused by it is 29%. What is puzzling is that the proportion of side effects in the group injected with the Sinopharm vaccine is only 28% to 29%.Equal to or lower than the side effects of adjuvant!
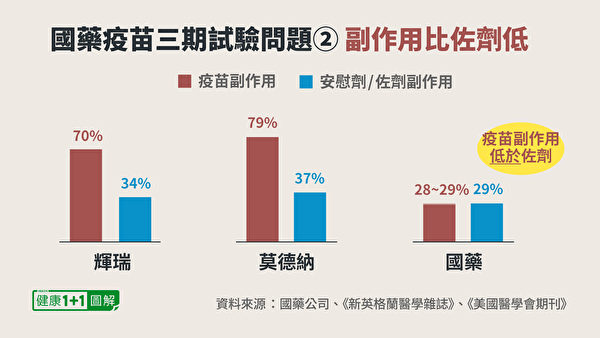
This can’t help but cause people to wonder, after the Sinopharm vaccine is administered, does an immune response really occur? Is there really enough antibodies in the body?
In addition, as mentioned earlier, the Phase III data of Kexing Vaccine are currently only published on the preprint website. It contains two test reports in Brazil and Chile. Among them, Chile reported that there were only more than 400 recruits in the third phase (usually tens of thousands of people were recruited for the third phase of the vaccine), and it did not give the overall protection of the vaccine.
HereNotEvaluate the quality of the vaccines of Kexing and Sinopharm, or the quality of other vaccines.But to exploreA pharmaceutical company, a country’s official medical institution, Is it responsible for the peopleBasic responsibility。
To make a vaccine that needs to be vaccinated for hundreds of millions of people, transparency and openness are of the utmost importance.
In recent days, many people posted on Weibo for help, telling that problems such as ear deafness, cerebral infarction, and heart disease occurred within a short period of time after being vaccinated with Kexing vaccine. These should be taken seriously by the government and the media as a rule.did not receiveAny concern. After the vaccine is widely administered, it is very possible that more serious side effects are found.AZ vaccineThere are also rare side effects of thrombosis, butThe media immediately paid attention, and the vaccine company and the government immediately issued several statements and conducted investigations.And thenConfirmed and listed as a side effect of the vaccine, The whole process keeps the public informed.How many people have side effects, the basic age and gender characteristics of each patient, the time of side effects, specific symptoms, and health status, The report is very detailed. This has nothing to do with the quality of the vaccine, but the basic responsibility of the government and pharmaceutical companies for the lives and health of the people.
In a chaotic world, if you have a healthy way, just lookHealth 1+1!
· The two countries with the highest vaccinations in the world have two polarities
· There are two risks of inactivated vaccines?Analysis of the three major issues of vaccines in China
·[Replay]The latest comparison of the seven major new crown vaccines is open
Editor in charge: Li Qingfeng
.