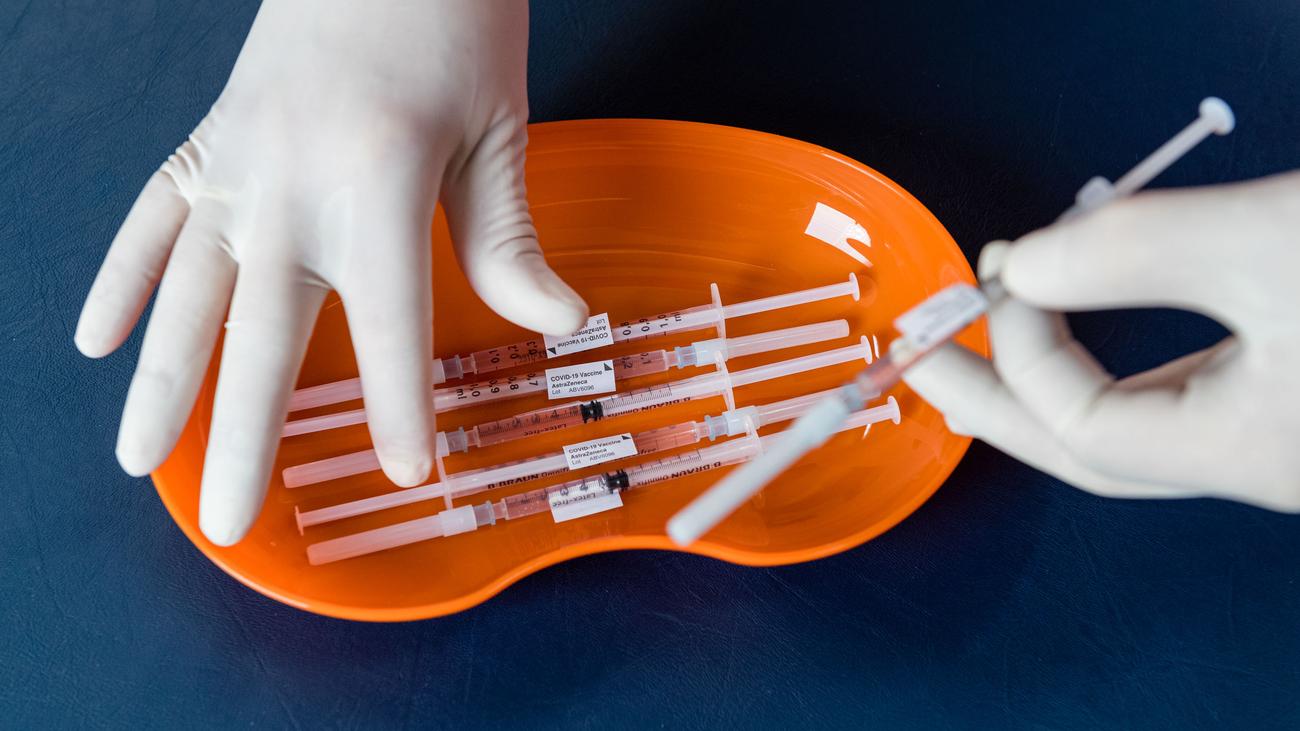
The corona vaccine from the Swedish-British pharmaceutical company AstraZeneca, Vaxzevria, no longer has EU approval. The withdrawal of market approval, which was decided in March, came into force on Tuesday, according to a document from the EU Commission. AstraZeneca requested this step itself, the company said. The background is a lack of demand.
More on the topic: Corona pandemic
RKI protocols: What we know about the RKI protocols
Vaccine damage: The vaccination and suspicion
Since the end of the pandemic, several variants of Covid-19 vaccines have been developed, which is why there is now a surplus of available updated preparations, AstraZeneca said. “This has resulted in a decline in demand for Vaxzevria, which is no longer manufactured or supplied.”
The EU Commission said in a statement that it was not unusual for companies to request the withdrawal of marketing authorization for medicines or vaccines for commercial reasons. The commission can confirm “that the decision is not based on doubts about the safety or effectiveness of the vaccine.”
AstraZeneca must provide information about side effects
The Bamberg Higher Regional Court recently ordered AstraZeneca to provide information about the possible effects of its corona vaccine. In the appeal process regarding alleged vaccine damage, AstraZeneca must disclose which effects and side effects of the vaccine the company was aware of between December 27, 2020 and February 19, 2024, a court spokesman said. The AstraZeneca vaccine was approved on December 27, 2020.
According to media reports, the drugmaker has admitted in court documents that the vaccine causes side effects such as blood clots and low platelet counts.
The corona vaccine from the Swedish-British pharmaceutical company AstraZeneca, Vaxzevria, no longer has EU approval. The withdrawal of market approval, which was decided in March, came into force on Tuesday, according to a document from the EU Commission. AstraZeneca requested this step itself, the company said. The background is a lack of demand.
Since the end of the pandemic, several variants of Covid-19 vaccines have been developed, which is why there is now a surplus of available updated preparations, AstraZeneca said. “This has resulted in a decline in demand for Vaxzevria, which is no longer manufactured or supplied.”