Access the article and all the contents of the site
with the dedicated app, newsletters, podcasts and live updates.
SPECIAL OFFER
BEST OFFER
ANNUAL
19€
For 1 year
CHOOSE NOW
MONTHLY
€1 PER MONTH
For 6 months
CHOOSE NOW
SPECIAL OFFER
BEST OFFER
ANNUAL
11,99€
For 1 year
CHOOSE NOW
MONTHLY
€2 PER MONTH
For 12 months
CHOOSE NOW
– or –
Subscribe by paying with Google
SPECIAL OFFER
Read the article and the entire website ilmessaggero.it
1 Year for €9.99 89,99€
or
€1 per month for 6 months
Automatic Renewal. Turn off whenever you want.
- Unlimited access to articles on site and app
- The 7:30 Good Morning newsletter
- The Ore18 newsletter for updates of the day
- The podcasts of our signatures
- Insights and live updates
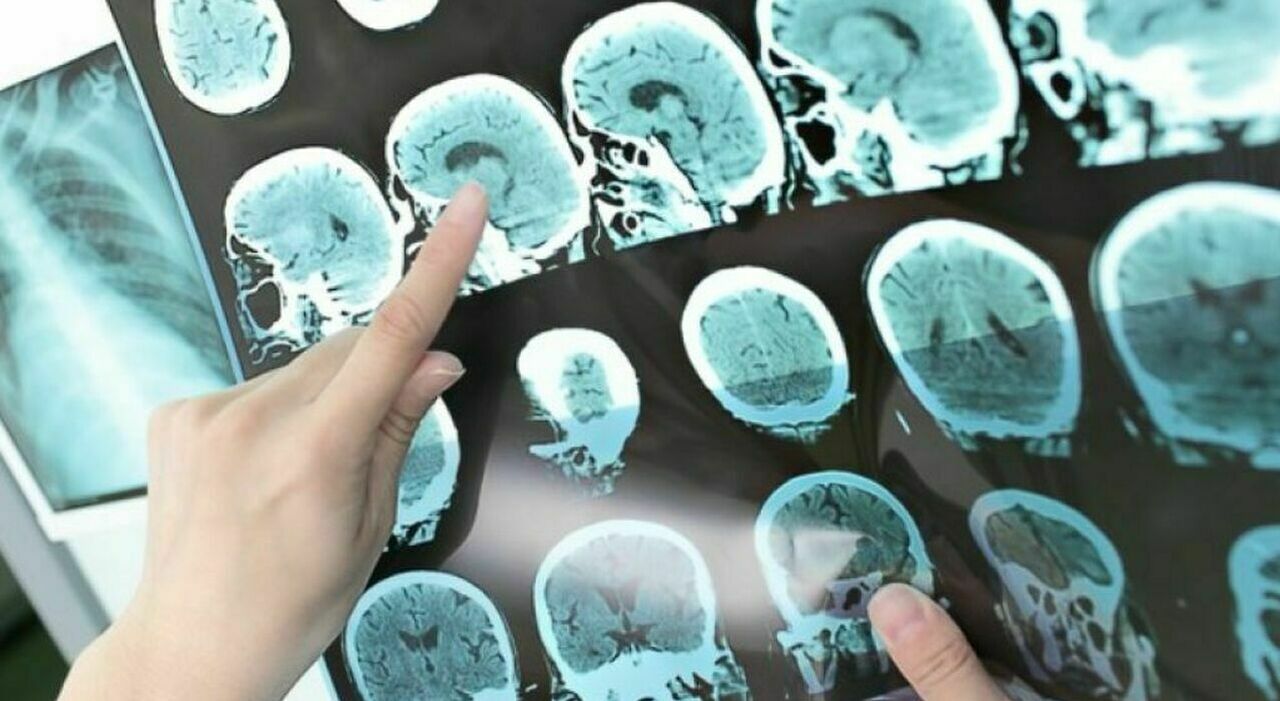
An experimental Alzheimer’s drug has shown promising results in a large trial of 1,700 patients over 18 months. The product, from Eli Lilly, stopped the cognitive decline of 47% of patients, all affected by the disease, for at least 1 year.
The «donanemab»-based medicine works by removing the amyloid plaque that forms in the brain of patients and is one of the markers of the disease: administered by infusion once a month, the product has been shown to remove most of the amyloid substance, so much so that 57% of the patients were able to discontinue the drug one year after the beginning of the therapy, and 72% of the volunteers achieved the same result after a year and a half.
Data disclosed by Eli Lilly have not yet been published, but they are so significant that the company will submit a request for rapid authorization of the drug to the Food and Drug Administration by June. However, three deaths occurred in the trial, the causes of which have not yet been fully ascertained, but two of them could have depended on cerebral micro-haemorrhages.
Alzheimer’s disease is the most common form of dementia, a general term that refers to loss of memory and other intellectual abilities so severe that it interferes with daily life. Alzheimer’s disease accounts for 50-80% of dementia cases.
Alzheimer’s disease is not a normal part of aging, although the greatest known risk factor is increasing age, and most people with Alzheimer’s disease are 65 and older. However, Alzheimer’s disease is not just a disease of old age. Up to 5 percent of people with the disease experience early-onset Alzheimer’s disease (also known as “early onset”), which often appears when a person is in their forties or fifties, or between their fifties and sixty years. Read more: Early Onset Alzheimer’s Disease and Risk Factors.
Read the full article
on The Messenger