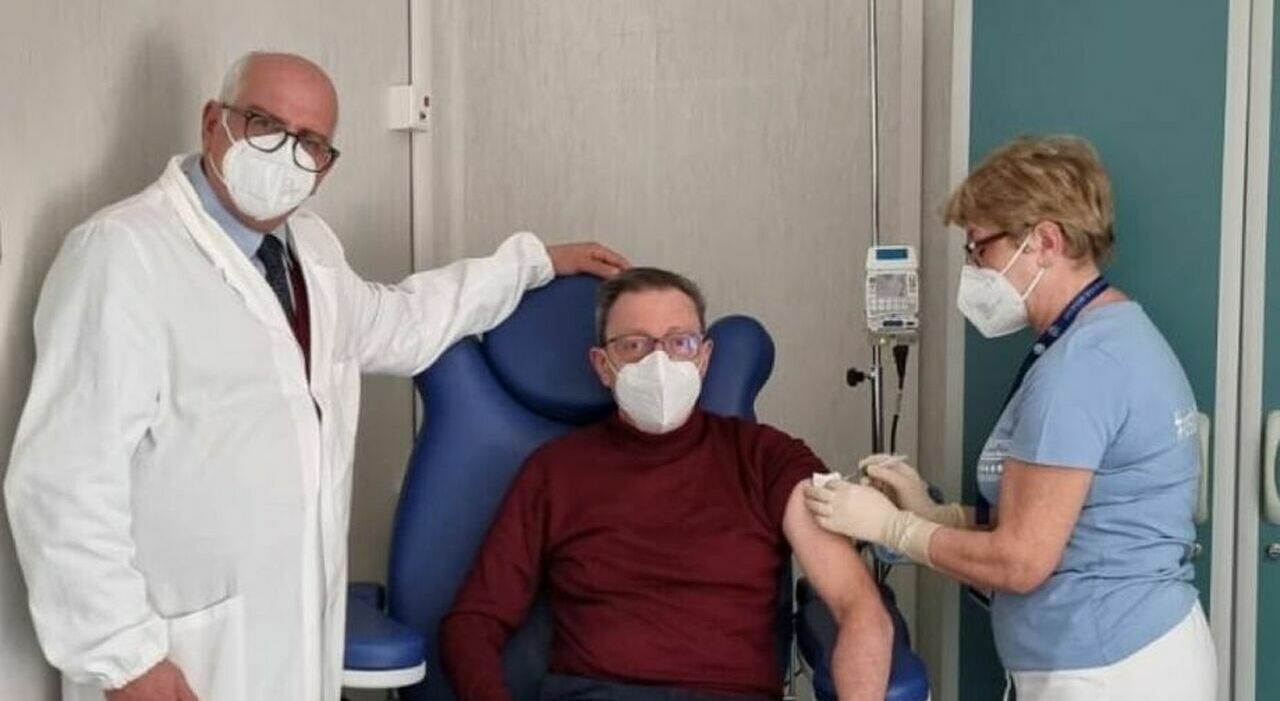
Important news in the field of fighting cancer. Alfredo De Renzis (71 years old) is the first Italian patient to receive the experimental mRNA anti-cancer vaccine for the treatment of melanoma. The dose was administered to him this morning at the National Cancer Institute “Pascale” Foundation in Naples, where the man has been under treatment since last September, followed by the oncologist Paolo Ascierto. The man, also a doctor, is participating in the phase 3 study of Moderna’s vaccine, the last step before the product can be approved by regulatory authorities.
INSIGHTS
Ascierto’s words: «Today is a great day», even if «it will take a few years before we have the results of this last phase […] our hope is to be able to give a new and more effective therapeutic option to as many patients as possible.”
The patient
«I accepted immediately. It seemed right to me for my role as a doctor to make a contribution to research. I have never been afraid.” These are the words of Alfredo De Renzis (71), a general practitioner in Carovilli, in the province of Isernia, the first Italian patient who was administered the mRNA anti-cancer vaccine for the treatment of melanoma this morning at the Pascale Tumor Institute in Naples.
De Renzis, married with two children, discovered two years ago that a melanoma was hidden behind a skin growth. After the initial treatment in Isernia he arrived in Naples, in the Pascale department coordinated by Dr. Paolo Ascierto. In September last year, inguinal lymph node metastases appeared. Operated in November by Alfonso Amore, member of Corrado Caracò’s team, he began treatment with Pembrolizumab on 15 December as part of the V904 study. Almost simultaneously with the start of immunotherapy, the proposal arrived to join phase III of Moderna’s first mRNA vaccine, the last step before the vaccine can be authorized by the regulatory authorities.
The experimentation
From what we learn, it seems that 18 other patients have already been selected to receive the same drug. This anti-melanoma vaccine, produced by the American company Moderna, is based on the same technology adopted for those against Covid.
As the oncologist explains, it uses synthetic mRNAs designed to “instruct” the immune system to recognize specific proteins, called neoantigens, which are the expression of genetic mutations that have occurred in diseased cells. Its purpose is not to prevent the disease, but to help and support the patients’ immune system to recognize and attack the tumor more effectively.
It is necessary to point out that, since it is a double-blind trial, the one injected into Alfredo could be a placebo dose. According to the protocol, in fact, neither the patient nor the oncologist knows what has been injected until the end of the trial.
The general director of Pascale, Attilio Bianchi, expresses strong emotion: «We are honored that Pascale is the first center in Italy to participate in the testing of the first mRNA vaccine against cancer. A completely new frontier is opening up and we are proud to be protagonists.”
The words of Vincenzo De Luca, president of the Campania Region, also arrived: «The administration of the first mRNA anti-cancer vaccine for the treatment of melanoma at the Pascale Cancer Institute in Naples is important news among many stupidities of political politics . But it is the confirmation of what I have always been saying: we have here, despite the difficulties, excellence of world value. Over the past two years, we have already invested 140 million euros in cancer research with the aim of identifying vaccines. We are starting to have the first results in this field. And this helps us in the battle to say that when we talk about healthcare, we forget to say that we are working in heroic terms with 15 thousand fewer employees and with tens of thousands fewer beds than in the North and with a division of the healthcare fund which is shamefully penalizing for the South. Despite everything, we reach peaks of absolute excellence. There is something to be proud of as Southerners, as Campanians and as Neapolitans.”
The point about anti-cancer vaccines
It is estimated that there are over 40 mRNA anti-cancer vaccines under study worldwide, while new indications for immunotherapy drugs already in use continue to increase. An example, given by the oncologist Ascierto himself, is pembrolizumab, a monoclonal antibody against PD-1, aimed at one of the “brakes” of the immune system, first approved for melanoma and last September authorized as a treatment for metastatic kidney cancer , for metastatic and perioperatory triple-negative breast cancer, for advanced endometrial and cervical cancers, for esophageal cancer and for some gastric and colon cancers.
«There are also combinations of immunotherapy – says the oncologist – as in the case of nivolumab and ipilimumab, approved and reimbursed by the National Health Service from 2022 for the treatment of metastatic non-small cell lung cancer, advanced kidney cancer in the front line of treatment, advanced esophageal cancer with chemotherapy progression, first-line pleural mesothelioma and some colorectal cancers. We have also received approval for the use of bispecific antibodies such as tebentafusp, in patients diagnosed with metastatic or unresectable uveal melanoma who present a particular antigen.”
To date, there are approximately 70 immunotherapy drugs under study, both in the preclinical phase (non-human trials) and in the clinical phase. In Italy alone there are around 200 ongoing clinical trials, of which 51 with active enrollment which in all respects represent a new therapeutic opportunity for patients.
© ALL RIGHTS RESERVED