daily economic news
2023-02-26 13:03:47
◎The “Report” shows that in recent years, several drugs for rare diseases have been included in the national medical insurance catalog every year, and high-value drugs for rare diseases have been included in the last two years. The number of innovative drugs for rare diseases in China will continue to increase in the future.However, in terms of genetic testing, special medical food, wearable devices, etc., domestic products and services for rare disease patients are still scarce
Every reporter Lin Zichen Every editor Dong Xingsheng
Since the release of the first batch of rare disease catalogs in China in 2018 and the Chinese definition of rare diseases, the domestic rare disease industry has been developing for five years. But in fact, as early as 2008, the international community has designated the last day of February as Rare Disease Day.
On February 25th, the eve of the 16th “International Rare Disease Day”, Frost & Sullivan (“Sullivan” for short) and Beijing Pain Challenge Public Welfare Foundation released the “2023 China Rare Disease Industry Trend Observation Report” online (the “Report”).
The “Report” shows that in recent years, several drugs for rare diseases have been included in the national medical insurance catalog every year, and high-value drugs for rare diseases have been included in the last two years. In the future, the number of innovative drugs for rare diseases in China will continue to increase.
However, in terms of genetic testing, special medical food, wearable devices, etc., domestic products and services for patients with rare diseases are still scarce.
70% of approved drugs for rare diseases have been insured, but far from enough
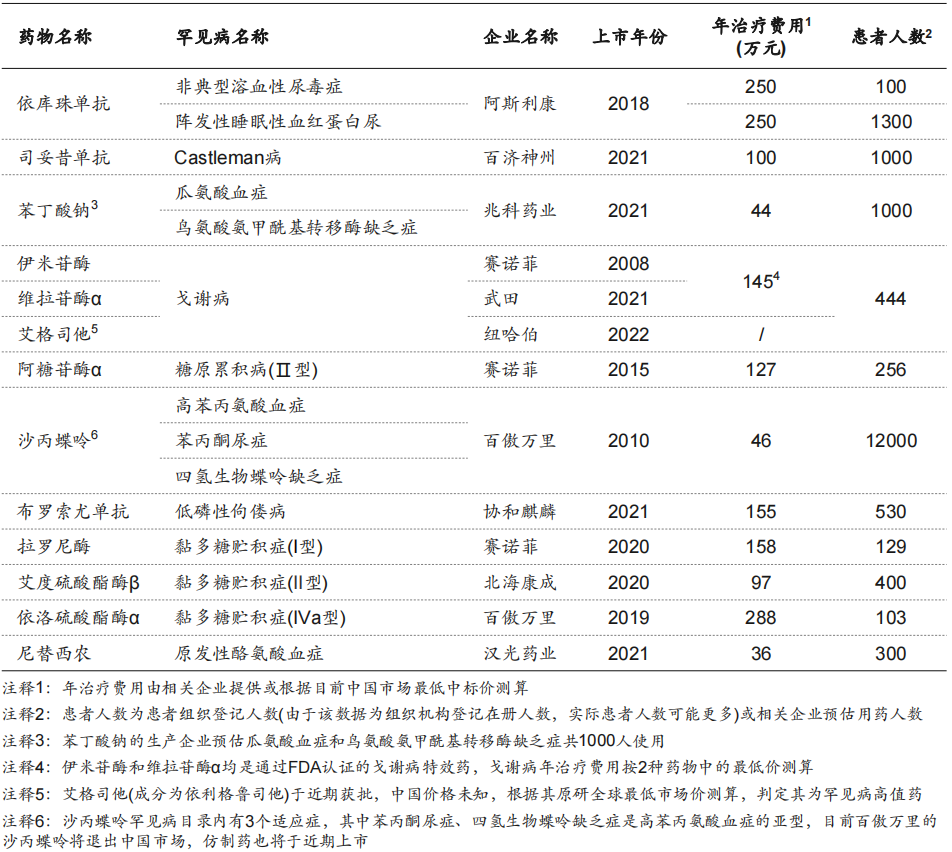
According to the “Report”, after the introduction of the “First List of Rare Diseases”, the number of rare disease drugs launched in my country has shown a significant upward trend, and the gap with the world is narrowing.
Up to now, based on the “First List of Rare Diseases”, 199 drugs have been launched globally, involving 87 rare diseases. Among them, 103 drugs are listed in China, involving 47 rare diseases; 73 of them are listed in China and included in the medical insurance, involving 31 rare diseases.
From 2019 to 2022, among the 121 rare diseases involved in the “First Batch of Rare Disease List”, there are 9 drugs for 6 rare diseases, 6 drugs for 6 rare diseases, and 7 drugs for 7 rare diseases. 7 drugs for 6 rare diseases, a total of 16 rare diseases and 29 rare disease drugs were included in the medical insurance through negotiation.
From the perspective of reimbursement, among the 73 rare disease drugs that have been insured, 17 are Class A drugs and 56 are Class B drugs. Class A drugs can be reimbursed in full, and Class B drugs need to pay a part of themselves. The reimbursement ratio varies with local policies and drugs, usually 70% to 80%.
However, the positioning of the national medical insurance as “not covering everything” means that the protection of rare diseases cannot be fully paid by the state. At present, 30 drugs for 24 rare diseases are not included in the medical insurance, and all the drugs for the treatment of 16 rare diseases (a total of 16 types) are not included in the medical insurance, and 13 of them are high-value drugs, and the annual treatment cost is high. It ranges from hundreds of thousands to millions, far exceeding the upper limit of 18,000 yuan per year for catastrophic health expenditures for domestic households.
In addition, although some drugs have been included in the medical insurance catalog, their indications for rare diseases are not covered. For example, although daratumumab for the treatment of primary light chain amyloidosis has been included in the medical insurance, the medical insurance payment does not include rare disease indications and can only be used for the treatment of multiple myeloma.
All therapeutic drugs are not included in the medical insurance of high-value drugs for rare diseases Image source: “Report”
This means that the core pain point to be solved at the local level is the protection of high-value drugs for rare diseases that are not included in the catalog. With the release of a series of systems such as the benefit list, localities are not allowed to establish other medical security systems beyond the scope of the basic system framework, posing new challenges to local rare disease protection.
Guo Jinchuan, director of information research at the Beijing Pain Challenge Public Welfare Foundation, analyzed the “Report” and said that new trends in future payment guarantees for rare diseases include risk sharing agreements, payments based on curative effects, and rare disease seed funds. Among them, the risk sharing agreement is jointly explored by local medical insurance departments and pharmaceutical companies. At present, its main application scenario is the payment and reimbursement process of rare disease special drugs in general insurance.
The rare disease market is heating up, with more than 10 listed companies involved
Although the high price of rare disease drugs is an insurmountable gap, from a global perspective, only 5% of rare diseases currently have effective treatments. The wish of more rare disease patients is not “medicine insurance”, but ” There are medicines available.”
For large multinational pharmaceutical companies whose patents have expired and suffered losses in revenue, they mostly expand their product pipelines through internal innovation, mergers and acquisitions, and R&D cooperation. Among them, the field of rare diseases with a large number of clinical needs to be met has become a hot investment track.
The “Report” shows that among the global pharmaceutical M&A projects in 2022, rare diseases are the areas with the most capital investment. Among them, Amgen acquired Horizon Therapeutics at a price of US$27.8 billion, setting the highest M&A price in the global pharmaceutical industry in 2022; Pfizer acquired Biohaven and Global Blood Therapeutics respectively, and cooperated with Beam Therapeutics to develop three drugs for liver, muscle and central nervous system In vivo base editing therapy for rare genetic diseases has the highest transaction frequency in this field.
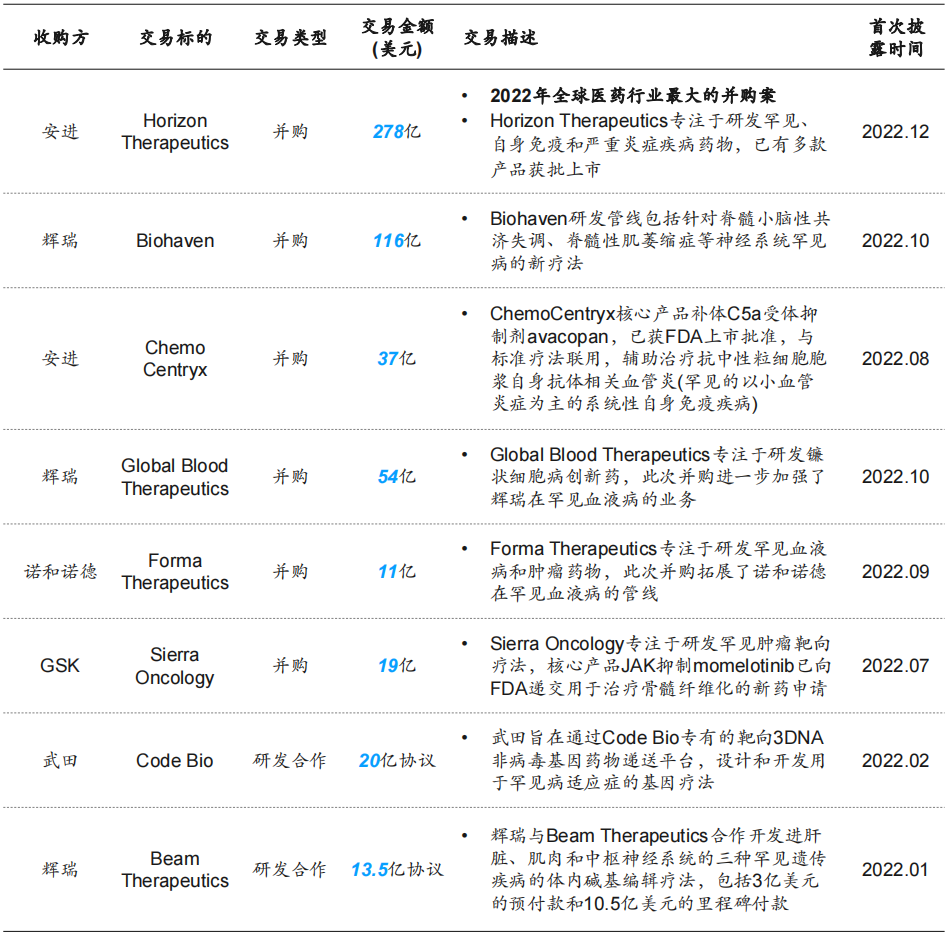
Pharmaceutical deals in rare disease sector, 2022 Source: “Report”
Before the release of the “First List of Rare Diseases” in 2018, domestic pharmaceutical companies had little involvement in the field of rare diseases, and the domestic rare disease drug market was dominated by multinational pharmaceutical companies. From 2018 to 2022, 27 drugs for rare diseases (excluding new indications) will be launched, of which only 4 drugs will be imported or imitated by domestic companies.
However, with the continuous development of the domestic innovative drug industry and the introduction of policies, more and more domestic pharmaceutical companies have begun to deploy in the field of rare diseases.
Since November 2018, the State Food and Drug Administration has successively released three batches of lists of overseas new drugs that are urgently needed in clinical practice. The varieties included in the lists can directly submit marketing applications, and CDE has established a special channel to speed up the review. There are a total of 81 varieties on the list of three batches of overseas new drugs urgently needed in clinical practice, more than half of which are rare disease drugs, and there are 37 rare disease drugs in the list.
As of February 2023, 81 drugs for rare diseases (excluding chemical generic drugs and biosimilar drugs) are in the stage of clinical trials and marketing applications, of which (67%) are independently developed or introduced by domestic pharmaceutical companies, involving more than 10.
In terms of rare diseases targeted by drugs, idiopathic pulmonary fibrosis, hemophilia, multiple sclerosis, Parkinson’s disease (juvenile onset, early onset), and neuromyelitis optica are currently the five most intensively developed rare diseases in China. The number of drugs in clinical trials and marketing applications are 20, 11, 8, 7, and 6, respectively.
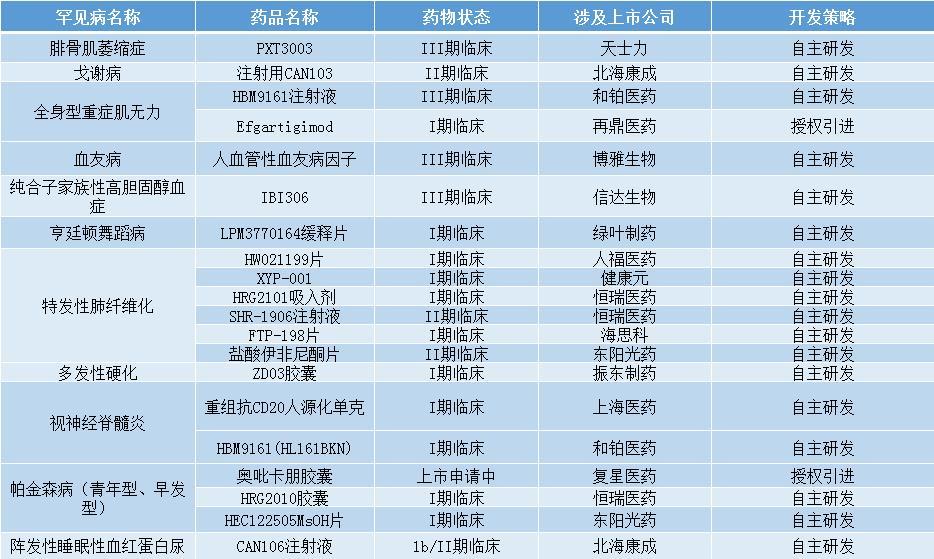
Drugs for rare diseases that are in the stage of clinical trials and marketing applications in China (only include self-developed or imported drugs by listed companies)
Source: Tabulated by reporter Lin Zichen according to the “Report”
There is still great potential in the fields of special medicine, food and gene sequencing
As the health problems of the rare disease population are paid more and more attention, technology companies around the world have deployed solutions other than drugs in the field of rare diseases, including data companies, diagnostic companies, wearable device companies, food companies, etc. However, in China, there are few products or services in these areas, and it is even impossible to obtain clear usage plans and purchase methods from doctors.
Taking food for special medical treatment as an example, food for special medical treatment refers to formula food specially processed and formulated to meet the special needs of nutrients or diet for people with food restriction, digestion and absorption disorders, metabolic disorders or specific disease states.
The “Report” shows that among the 121 rare diseases involved in the “First List of Rare Diseases”, 32 rare diseases require the use of formula foods for special medical purposes for related treatment, and 18 rare diseases require timely, life-long, Use special medical food in sufficient quantity. Different from the impression of food-assisted treatment in public perception, food for special medical treatment is the main and core treatment method in the clinical treatment of such rare diseases. If not treated in time, patients will face developmental delay or regression, and even death and disability. Serious consequences.
However, FSMP needs product registration and approval before it goes on the market in my country, and the current product supply is difficult to meet the purchase needs of patients. The data shows that after the implementation of the FSMP registration management system in my country, as of October 2022, a total of 92 FSMPs have been approved, and only 3 products for phenylketonuria, a rare disease, have been approved.
In addition, the ecology of technological innovation for rare diseases is relatively loose and fragmented.
Xue Qun, founder, chairman and CEO of Beihai Kangcheng (HK01228, stock price 2.30 Hong Kong dollars, market value 976 million Hong Kong dollars), said at the meeting that clinical genetics consultation for rare disease patients in China has not yet been fully developed, but the current domestic generation, The second-generation gene sequencing has been relatively sound. Pharmaceutical companies can cooperate with gene sequencing companies to provide more information for patients with rare diseases and their families. This is an early warning and risk prediction link that is currently lacking in the industrial ecology, and it is also an important factor that contributes to the formation of a closed-loop industry. link.
Wang Jue, head of business development of Insilicon in China, said that the company hopes to use artificial intelligence technology to empower the research and development of rare disease drugs. For example, INS018_055 is a potential first-in-the-world candidate drug for the treatment of idiopathic pulmonary fibrosis discovered by the end-to-end artificial intelligence platform of Insilicon.
Wang Jue said that the drug uses artificial intelligence technology to discover new targets and further generate small molecule drugs. It has completed phase I clinical trials in New Zealand and China, and will soon conduct phase II clinical trials in China and the United States.
Cover image source: Visual China-VCG21gic6332800