Access the article and all the contents of the site
with the dedicated app, newsletters, podcasts and live updates.
SPECIAL OFFER
BEST OFFER
ANNUAL
19€
For 1 year
CHOOSE NOW
MONTHLY
€1 PER MONTH
For 6 months
CHOOSE NOW
SPECIAL OFFER
BEST OFFER
ANNUAL
11,99€
For 1 year
CHOOSE NOW
MONTHLY
€2 PER MONTH
For 12 months
CHOOSE NOW
– or –
Subscribe by paying with Google
SPECIAL OFFER
Read the article and the entire website ilmessaggero.it
1 Year for €9.99 89,99€
or
€1 per month for 6 months
Automatic Renewal. Turn off whenever you want.
- Unlimited access to articles on site and app
- The 7:30 Good Morning newsletter
- The Ore18 newsletter for updates of the day
- The podcasts of our signatures
- Insights and live updates
The countdown to 2024 has started. In just under a year, the treatment of one of the most severe forms of age-related macular degeneration will be available: the FDA approves Syfovre. The Irccs Bietti Foundation participated in the Phase 3 international studies of this treatment: the first and only one recognized by the US government agency. A disease that affects 5 million people in the world and which leads to the progressive and irreversible loss of vision: it is geographic atrophy (GA), an advanced form of senile macular degeneration (AMD).
I study
Research has made progress for the treatment of this disabling pathology and the Bietti IRCCS Foundation – the only Scientific Hospitalization and Care Institute dedicated to ophthalmology in Italy – has given its valuable contribution by actively participating in international studies of Phase 3, discovering an innovative treatment for geographic wasting, the first and only one approved by the Food and Drug Administration. An important discovery that can lead to saving the sight of millions of people.
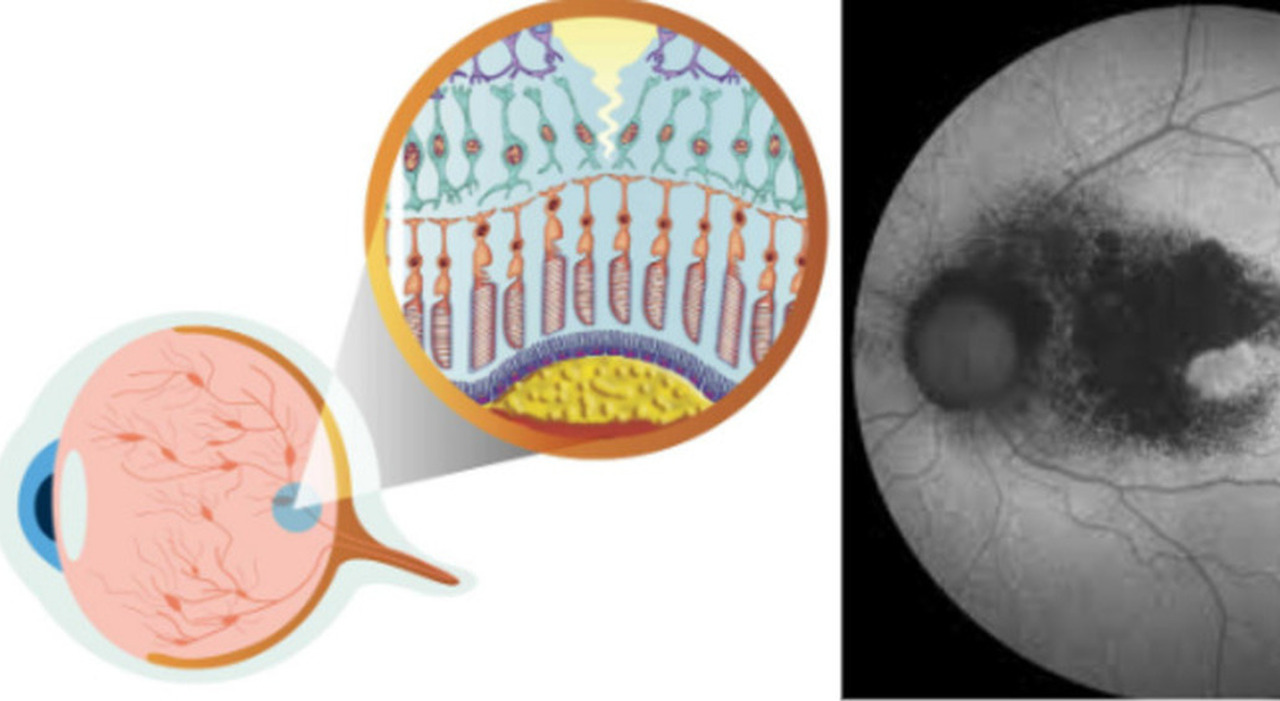
«The loss of vision caused by GA seriously compromises independence and quality of life, making it difficult to participate in daily activities – explains Dr. Monica Varano, scientific director of IRCCS Bietti -. In people with GA, photoreceptors, which are light-sensitive cells, deteriorate in the macula, a central portion of the retina responsible for central vision and color perception. This damage begins as small areolas that develop into larger areas; a person with early age-related macular degeneration (AMD) may experience problems with reading or night vision. Eventually, if the disease progresses to advanced stages, permanent blind spots (scotomas) will develop in the center of the visual field.
What causes the disease
The cause of AG is thought to be multifactorial, with numerous environmental and genetic risk factors. Dysregulation of the complement cascade, an important part of the body’s immune system, plays a critical role. “Excessive activation of the complement cascade causes the destruction of healthy cells, which can lead to the onset or progression of many diseases including GA – continues Dr. Monica Varano. – Precisely the different phases of the complement cascade have been the main target of international trials in recent years. One such molecule being studied was Pegcetacoplan, also known as APL-2, (Apellis Pharmaceuticals, Waltham, MA, USA) which inhibits the cleavage of factor C3 into C3a and C3b.”
The approval
On February 17, 2023, the US Food and Drug Administration (FDA) approved SYFOVRE – Pegcetacoplan intravitreal injection for the treatment of geographic atrophy (GA) secondary to age-related macular degeneration. The approval of SyfovreE is based on the positive results of the 24-month Phase 3 OAKS and DERBY studies on a large and representative patient population, international studies in which the Bietti Foundation actively participated.
Syfovre is the first and only FDA-approved treatment for geographic atrophy.
«In particular, Syfovre is approved for patients with GA with or without subfoveal involvement – specifies the scientific director of the Bietti Foundation -. In the OAKS and DERBY studies, Syfovre reduced the rate of GA lesion growth compared to the control group and demonstrated increasing treatment effects over time, with the greatest benefit (up to 36% reduction in lesion growth with monthly treatment in DERBY) which occurred between months 18-24. The safety profile of Syfovre is well demonstrated after 12,000 injections,” concludes Dr. Varano. In turn, the European Medicines Agency – EMA – is reviewing a marketing authorization application for Syfovre, with a decision expected in early 2024.
Read the full article
on The Messenger