Elena Meli
From Bambino Gesù in Rome, data demonstrating the effectiveness of CRISPR-Cas9 therapy to treat two genetic blood diseases: optimal and stable results for over 90 percent of patients
Correcting DNA with ultra-precise “molecular scissors” to cure diseases that depend on genetic defects such as thalassemia and sickle cell anemia, the two most widespread hereditary blood diseases in the world: today it is possible, as demonstrated by the results of two newly published studies on New England Journal of Medicine in which the Bambino Gesù Hospital in Rome participated. This is “a milestone in the treatment of these diseases”, as underlined by Franco Locatelli, head of the clinical and research area of Oncohematology, cell therapy, gene therapies and hematopoietic transplant of the Bambino Gesù and coordinator of the investigation into thalassemia: thank you to the “molecular scissors” 91 percent of patients with thalassemia no longer have to undergo transfusions, while 97 percent of people with sickle cell anemia have not had crises with occlusion of blood vessels for a year.
Correct the DNA
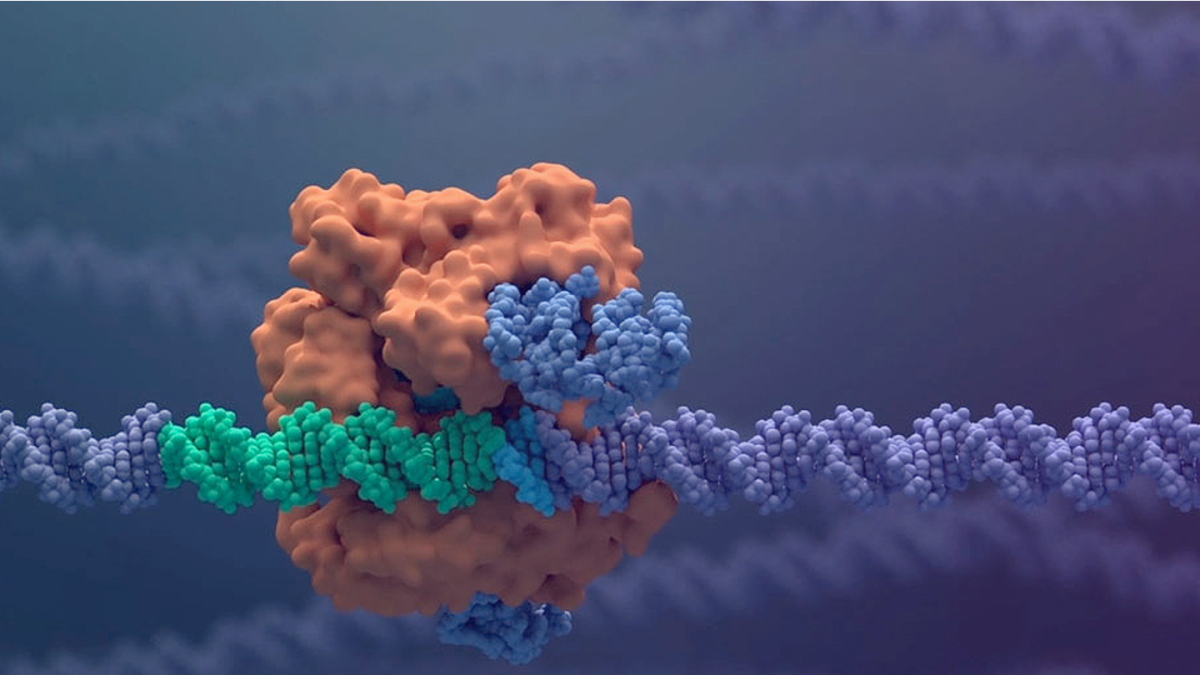
The “molecular scissors” used in the two studies are based on the use of the Cas9 protein, capable of cutting specific DNA sequences, associated with RNA segments which, like a compass, indicate the specific genetic target to hit: the set of Cas9 and of RNA fragments, associated with other proteins, constitutes the CRISPR-Cas9 system, a “corrector” that allows precise sequences of a cell’s genome to be cut or modified. This technology can therefore potentially solve diseases that depend on a defective gene: CRISPR-Cas9 is programmed to recognize and cut the specific anomalous gene sequence, the cells of the sick person are taken and treated in the laboratory with this system; then, the corrected cells are reintroduced into the organism so that they reproduce in place of the defective ones. In the case of thalassemia and sickle cell anemia, the target of CRISPR-Cas9 is BCL11A, a gene fundamental in the synthesis of hemoglobin, the protein that transports oxygen in the blood. The hemoglobin present from birth onwards contains protein chains called alpha-beta, the fetal one contains alpha-gamma chains, but in people with thalassemia or sickle cell anemia the problem is in the production of beta chains: in thalassemia the beta chains are produced in low quantities or completely absent, so there is very little hemoglobin in circulation and patients must regularly undergo transfusions; in sickle cell anemia the beta chains are present, but they are abnormal and change the shape of the red blood cells, making it difficult for them to pass through the blood vessels. To overcome the defects of the beta chains, it was then decided to “switch off” the BCL11A gene with molecular scissors, which is responsible for the transition from the production of fetal gamma chains to beta chains after birth: once BCL11A had been silenced with CRISPR-Cas9 , the body resumes producing fetal hemoglobin with alpha-gamma protein chains, eliminating the problems that arise from hemoglobin with defective beta chains.
Results in over 90% of cases
The approach works: three years ago researchers published data obtained on a patient with thalassemia and one with sickle cell anemia, demonstrating the feasibility of the strategy. In practice, the stem cells responsible for the production of blood cells are taken from the patients’ bone marrow, corrected with CRISPR-Cas9 and then transferred back into the body, after eliminating the bone marrow with a specific therapy so that the modified cells have space to proliferate and thus resolve the disease. This is exactly what happened in the patients involved in the two new studies CLIMB-111, on 52 people with thalassemia, and CLIMB-121, on 44 cases of sickle cell anemia. In the case of thalassemia, 16 months after therapy, 91 percent of patients no longer needed transfusions and after a few more months everyone was able to do without them; Four years have now passed since the first treatments, but the presence of the modified cells has remained stable, demonstrating that the effect of the infusion of modified cells is long-lasting. The same goes for patients with sickle cell anemia, for whom the objective was not independence from transfusions but the absence for at least a year of episodes in which the vessels become occluded due to the sickle-shaped red blood cells: the 97 percent of treated cases went at least 12 months without having even a crisis. All with an excellent safety profile, because the patient is a self-donor and therefore there are no problems that would arise from a donor marrow transplant.
Italian excellence
The therapy is approved by the Food and Drug Administration and the European Medicines Agency for patients over 12 years of age but, given the safety data, two new trials are already underway at Bambino Gesù which to date have involved two young patients for both diseases, with encouraging results. The Roman hospital, which coordinated the international study on thalassemic adults and was the center with the largest number of patients involved in the sickle cell anemia trial, therefore confirms its excellence in innovative therapies: as Franco Locatelli observes, these data they are «a sort of milestone for the change in therapeutic scenario and the definitive curative potential of these two pathologies so widespread in the world. A result that demonstrates once again the ability and determination of the Bambino Gesù Pediatric Hospital to invest in innovative therapies capable of changing the natural history of such complex diseases. These studies demonstrate how the Hospital pays attention to everything that can change the probability of survival and the quality of life of patients suffering from genetic diseases.”
April 25, 2024 (changed April 25, 2024 | 09:50)
© ALL RIGHTS RESERVED